Our Breakthroughs
Navigene’s relentless pursuit of multidisciplinary innovation has produced notable breakthroughs, some already validated and commercialized for realworld impact.
Expanding the metabolic disorders panel to 115 disorders using dried urine spot

Traditional newborn screening uses a painful heel-prick blood sample to test for up to 50 metabolic disorders and often requires a second, urine-based confirmatory test — increasing cost, anxiety, and turnaround time. Early discharge and deliveries in remote settings further reduce feasibility.
Navigene Baby Screen is a revolutionary urine-based, non-invasive test that screens newborns for 110 genetic and metabolic disorders from as early as day 2 of life, using a simple Urine sample collected on a filter-paper. It uses Planar diagnostics to analyze 250+ unique markers in the urine for pinpoint and accurate diagnosis of metabolic disorders. It also provides built-in confirmatory results for ~80 % of conditions, reducing false positives and eliminating follow-up testing in most cases.
Developed in India, Navigene is the world’s first and most comprehensive non-invasive newborn screening platform, already trusted across India, Southeast Asia and Latin America.
Single step DBS test for clinically relevant hemoglobinopathies on an Open HPLC system
HPLC remains the gold-standard for diagnosing hemoglobinopathies but relies on venous whole-blood collection and cold-chain logistics, limiting its use in remote regions. Dried blood spot (DBS) sampling provides a simple finger-prick alternative, requiring only a few drops of blood that can be dried on filter paper and shipped at ambient temperature.
While DBS-HPLC is widely used in newborn screening, its use in children and adults is virtually unexplored, and existing closed-system HPLCs (like Bio-Rad) are not compatible with DBS samples from these age groups. To bridge this gap, we successfully developed a proprietary DBS-based protocol using open HPLC systems, specifically optimized for newborns, children, and adults—enabling reliable, quick and comprehensive diagnosis of hemoglobinopathies even in low-resource settings.
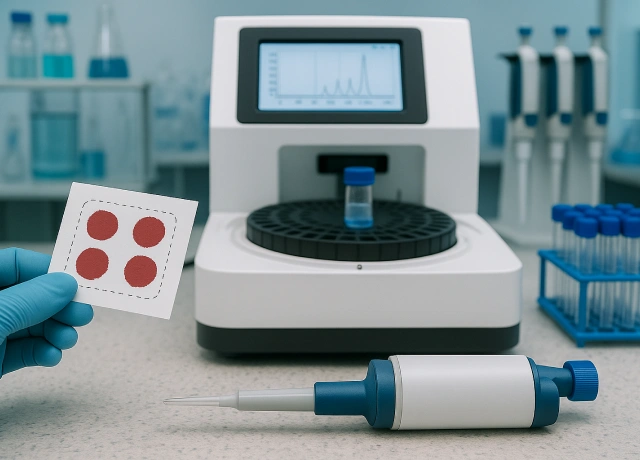
Significant reduction in HPLC sample processing time through proprietary mobile-phase chemistry and optimized column reengineering modifying flow rates

Our in-house developed, validated and NABL-accredited DBS-HPLC test for hemoglobinopathies (across all age groups) is not only highly accurate and comprehensive but also designed for high-throughput mass screening. Over three years, we engineered proprietary optimizations — including changes to column chemistry, mobile phase molarity/pH, and gradient flow — enabling the assay to deliver equivalent results in half the time of traditional closed-system HPLC platforms.





